Special Recipe
by Andy Boyd
Today, a special recipe. The University of Houston presents this series about the machines that make our civilization run, and the people whose ingenuity created them.
Simple recipes are the best. Here's one you can try at home, though you should probably put it together in your garage.
Start with fifteen gallons of water. To that, add nine cups of frozen nitrogen. Together, they'll give you a nice fog rolling over the side of your mixing pot. Finally, add one-and-a-half gallons of graphite, the type of carbon you find in pencils. Mix well. Congratulations. You're 96 percent of the way to making a 150 pound human.

Stirring together a human. Photo Credit: Andy Boyd
Broken down to its basic ingredients, the human body is strikingly simple. To finish our body-building exercise, we'd need to add about a cup each of calcium, phosphorous, potassium, sulfur, and salt. Pinches of just a few additional ingredients and we'd be done.

The simple recipe for a human. Photo Credit: Andy Boyd
Of course, how we mix things together is vitally important. It's not just what goes into the mix, but how the various components are linked into complex molecules. But any way you look at it, it's amazing that a small collection of basic ingredients can give rise to anything as remarkable as life. And not just human life. All life on earth ' animal and plant ' arises from these same ingredients put together in essentially the same way. There are variations, of course. Animals with bones, for example, have proportionately more calcium. But the similarities among living things far outweigh the differences. And we're led to ask. Must this be the case, or is it possible for life to form in some radically different way?
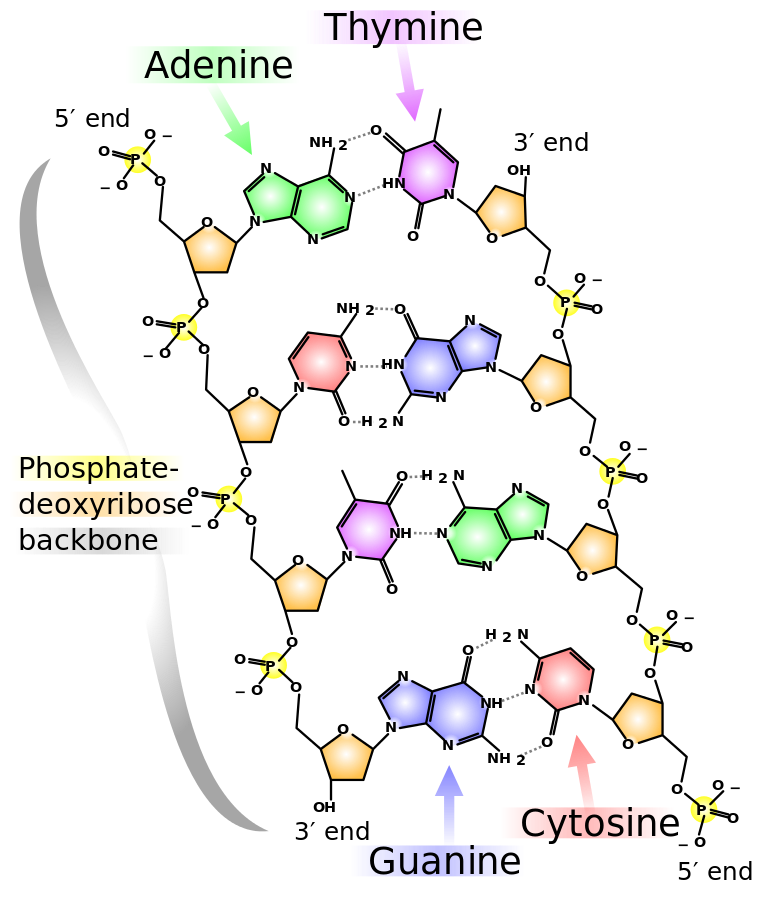
Chemical structure of DNA. Photo Credit: Wikimedia
It's an interesting question in general, but especially to planetary scientists. Some have speculated that life could arise in lakes of liquid ethane found on Saturn's largest moon, Titan. If so, the life found there would almost certainly have a very different biochemistry than life on earth.
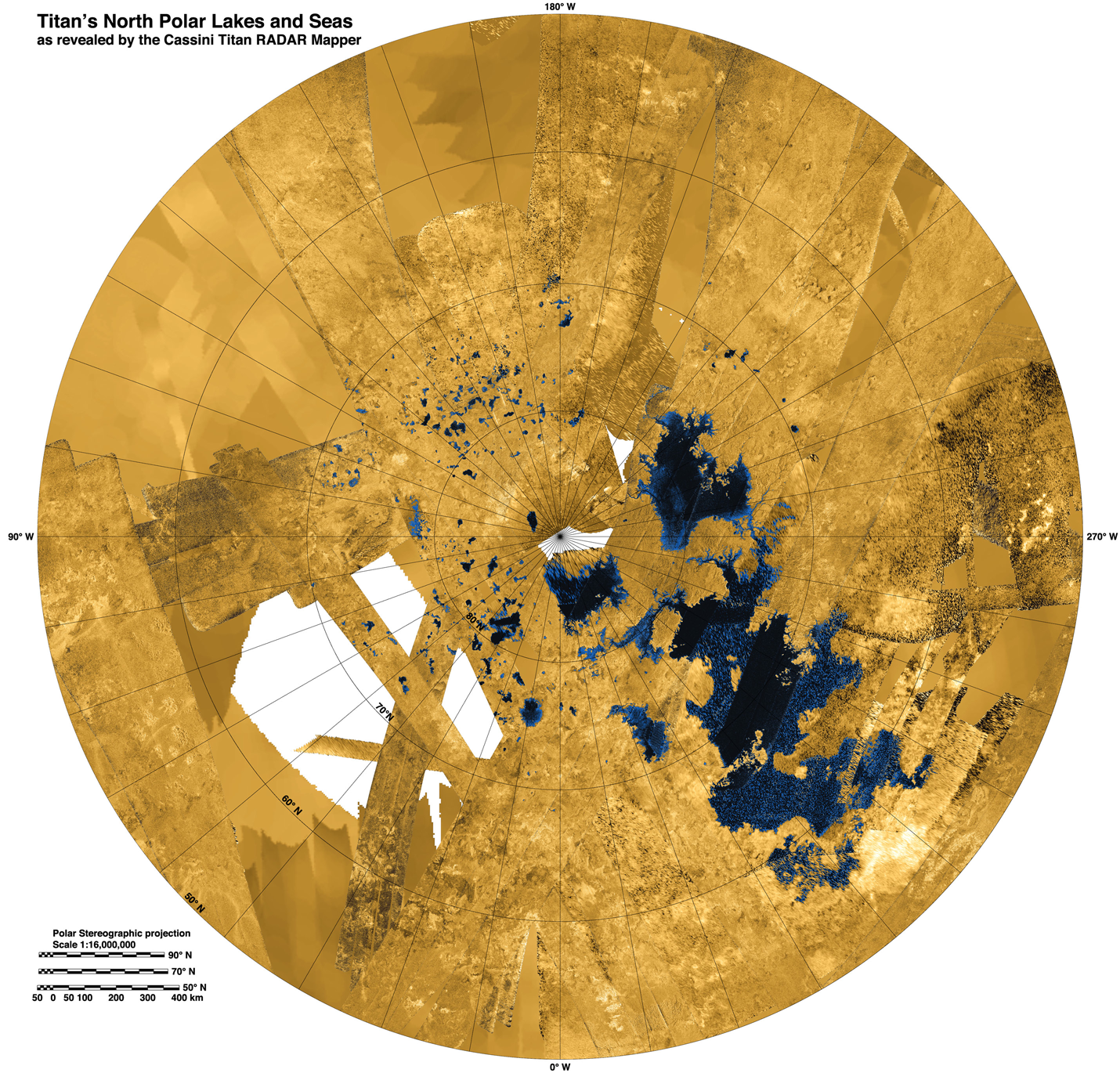
This colorized mosaic from NASA's Cassini mission shows the most complete view yet of Titan's northern land of lakes and seas. Photo Credit: Wikimedia
The phosphorus found in our bodies is chemically quite similar to arsenic, and it's been speculated that life could arise by exchanging one for the other. If so, how different would the resulting life be from life as we now know it?
And what about intelligent life? Somehow, when put together in just the right way, the makings of the human body give rise to the complex mental activities we're all so intimately familiar with. Could other biochemistries lead not just to life, but to intelligent life? Or here's a thought: could they lead to life more intelligent than our own?
It's fun to speculate, but as far as we know, water, carbon, nitrogen and the like form the foundation for all living things. From the flowers in our gardens to the dogs who anxiously await our return home, all life springs from the same elemental origins. We may someday discover some altogether different form of life, but I'm not holding my breath. What we have here and now is a rich enough recipe for many lifetimes.

A tricolor pansy. Photo Credit: Wikimedia

The happiest Golden Retriever in the world. Photo Credit: Wikimedia
I'm Andy Boyd at the University of Houston, where we're interested in the way inventive minds work.
(Theme music)
The ingredients mentioned here are elements except for water (comprised of the elements hydrogen and oxygen) and salt (comprised of the elements sodium and chlorine).
For a related episode, see INSIDE THE MACHINE.
Building Blocks of Life. From the Arizona State University School of Life Sciences website: https://askabiologist.asu.edu/content/atoms-life. Accessed January 24, 2017
Hypothetical Types of Biochemistry. From the Wikipedia website: https://en.wikipedia.org/wiki/Hypothetical_types_of_biochemistry. Accessed January 24, 2017.
This episode was first aired on January 26, 2017