Everything There Is--By The Numbers
by Andrew Boyd
Today, everything there is — by the numbers. The University of Houston's College of Engineering presents this series about the machines that make our civilization run, and the people whose ingenuity created them.
A flower. A rock. A toaster oven. All very different things. And all made of very much the same stuff.
We learned about the basic building blocks of the universe in middle school — atoms. They're made up of just three different particles — protons, neutrons, and electrons. The details have changed a bit over the years. Electrons no longer whirl about an atom's nucleus like planets around a star. They now play hide and seek in probability clouds. And we can divide these tiny particles into smaller fragments if we like. But protons, neutrons, and electrons are all we need for a good look at everything we encounter in our daily lives. Just three particles — the ingredients of flowers, rocks, toaster ovens, and everything else.
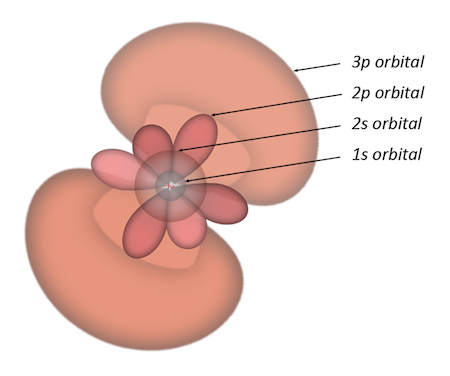
The electron orbitals of a neon atom (Lawrence Berkeley National Laboratory)
Our world starts to take shape as we mix these ingredients together. One proton plus one electron gives us a hydrogen atom. Six protons, six neutrons, and six electrons and we have a carbon atom. But not every combination of particles holds together. Altogether, just under 100 types of atoms, or elements, exist in nature. No matter where you go in the universe, you'll only find these 100 or so varieties of atoms.
Not in equal proportions, of course. The lightest two, hydrogen and helium, make up 98 percent of what we see in the universe. They have an unfair advantage in that they've been around since shortly after the Big Bang. Most of the other elements had to wait for giant nuclear reactors known as stars to churn them out. As astrophysicist Carl Sagan put it, "...the calcium in our teeth, the iron in our blood ... we are made of starstuff."

The image is from the European Space Agency. It is listed as the LH 95 star forming region of the Large Magellanic Cloud. The image was taken using the Hubble Space Telescope. (European Space Agency/Wikipedia)
Most of the hydrogen and helium in the universe is still trapped in stars. Here on earth the outer crust is dominated by elements like oxygen and potassium. Altogether, a mere 8 elements make up 99 percent of what we find here on earth — a testament to simplicity.
The story starts to grow in complexity when we look at molecules — atoms that have joined forces. We all know the chemical formula for water: H2O — two hydrogen atoms bonded with one oxygen atom. But molecules can be made of any number of atoms. And molecular properties don't depend just on the atoms, but on the way they're hooked together. Human DNA is a molecule made up of only five different elements: carbon, hydrogen, oxygen, nitrogen, and phosphorous. But the number of individual atoms runs into the hundreds of billions. And as we know, two strands of DNA that are 99.9 percent identical can behave very differently. When atoms band together, the possibilities are almost limitless.
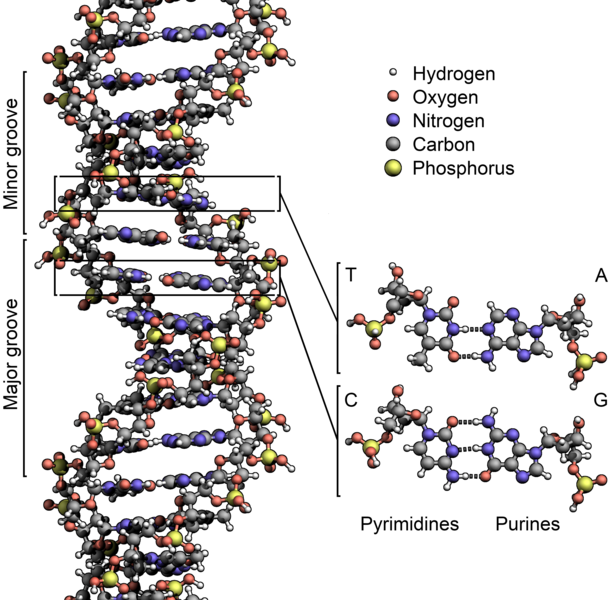
The structure of DNA showing with detail showing the structure of the four bases, adenine, cytosine, guanine and thymine, and the location of the major and minor groove. (Zephyris/Wikipedia)
But it all starts with a simple trinity of particles — protons, neutrons, and electrons. Such a wildly rich universe; such a surprisingly humble foundation.



From left to right: A Nelumno nucifera flower in a botanical garden in Adelaide (Peripitus/Wikipedia), an unknown white rock (Jon Zander/Wikipedia), and a toaster oven (Wikipedia)
I'm Andy Boyd at the University of Houston, where we're interested in the way inventive minds work.
(Theme music)
Notes and references:
When artificially created elements are included, the list of elements grows to 118.
Abundance in the Universe of Elements. From the website: http://periodictable.com/Properties/A/UniverseAbundance.html. Accessed May 20, 2014.
Nucleosynthesis. From the Wikipedia website: https://en.wikipedia.org/wiki/Nucleosynthesis. Accessed April 17, 2014.
Ten Most Abundant Elements in Earth's Crust. From the Jefferson Laboratories website: http://education.jlab.org/glossary/abund_ele.html. Accessed April 17, 2014.
This episode first aired on May 22, 2014.