Inventing Benzene
Today, a murder inquest, and a serpentine ring, touch a young man's mind. The University of Houston's College of Engineering presents this series about the machines that make our civilization run, and the people whose ingenuity created them.
Physicist Hans von Baeyer tells a strange story about the benzene molecule. It begins in 1850 with a young architecture student, Friedrich Kekule, testifying before a grand jury in Giessen, Germany. The charred body of a neighbor lady had been found in her room. People thought she'd been the victim of spontaneous combustion, brought on by drinking too much liquor.
The great chemist Justus von Liebig testified at the trial. He made it quite clear that the lady would have died long before she'd drunk enough alcohol to make her flammable. Then Kekule's testimony incriminated a servant who'd been stealing from the lady. He identified her distinctive ring, which turned up in the servant's possession. Together, Liebig's and Kekule's testimony convicted the scoundrel of murdering the lady.
The trial left its mark on the young Kekule. He gave up architecture and took up the study of chemistry with Liebig. The lady's odd ring also lingered in Kekule's mind -- it had carried the old alchemy seal of two intertwined serpents biting each other's tail.
Fifteen years later, Kekule worked with Liebig on a new chemical called benzene. Logic dictated that it must be an arrangement of six carbon, and six hydrogen, atoms. But how could you arrange such a molecule without violating the rules of chemical valence? It didn't seem possible.
Kekule dozed in his chair by the fire, trying to solve the riddle. As he nodded, he dreamt of the twining serpents on that old ring, whirling in the flames. Suddenly, in the dream, the serpents caught each other's tail and formed a circle. Kekule saw the answer. The carbon atoms formed an hexagonal ring with alternating single and double bonds. Each one held its own hydrogen atom -- "like charms on a bracelet," says von Baeyer. It was a structure utterly alien to anything else in chemistry.
We're seldom given such a clear account of the moment an idea reveals itself. But in this case the author's great-grandfather worked with Kekule and handed down a rare insight. We're shown two features of inventive thought: One is that the inventor can bring outside abilities to a field. Kekule had an architect's spatial and structural sense -- a place to stand outside of chemistry -- another way to look at things.
But he was also able to place a problem in his subconscious mind and turn his dreams loose on it. Pure invention breaks the thread of logic. It arises where we least expect it. Pure invention is the fruit of recognition rather than deduction.
I'm John Lienhard at the University of Houston, where we're interested in the way inventive minds work.
(Theme music)
Von Baeyer, H.C., A Dream Come True. The Sciences, Jan./Feb., 1989, pp. 6-8.
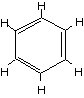
The benzene molecule