John Lennard Jones
Today, who was Lennard Jones? The University of Houston's College of Engineering presents this series about the machines that make our civilization run, and the people whose ingenuity created them.
All life seems to be attraction and repulsion. We seek each other out, then push each other away. Those forces set workable distances. Friendships have stability when the distance is agreeable to both parties. Good fences really do good neighbors make.
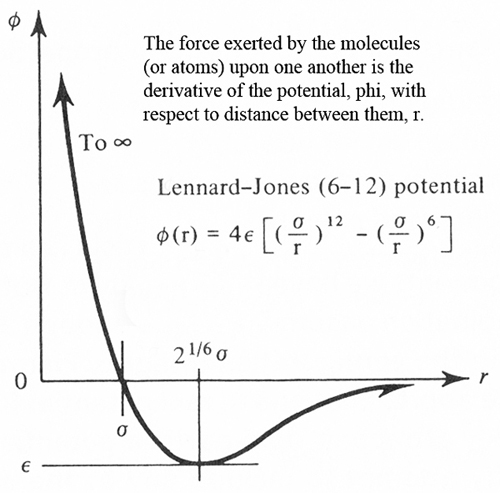
Atoms and molecules are like that. The oxygen and nitrogen molecules around us are in constant motion. They attract one another from a distance and that attraction strongly increases as they draw close. But then, they suddenly develop a huge repulsion.
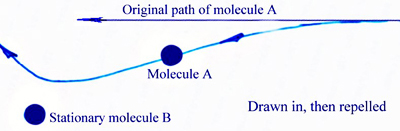 All those teeming molecules travel near the speed of sound, yet never hit each other. That's because the repulsion is so strong when they get close, they can't ever touch. The attraction force, though it reaches out some distance, is pretty weak. The close up repulsion is much stronger.
All those teeming molecules travel near the speed of sound, yet never hit each other. That's because the repulsion is so strong when they get close, they can't ever touch. The attraction force, though it reaches out some distance, is pretty weak. The close up repulsion is much stronger.
The two atoms in, say, an oxygen molecule are actually bonded to one another -- constantly drawn close, then pushed apart. They vibrate together as they fly about in their role as a molecule. That sounds rather like coworkers or couples, in fact.
Now, a curious catch in all this: When gases are thin enough and warm enough, their vibrating molecules move so fast that they almost never affect one another. We then call the gas ideal or perfect. And we can we can describe it with the almost childishly simple Ideal Gas Law (pV = RT). I suppose that too has its analogy in human affairs. It is an ideal devoid of any messy interactions -- not like a world any of us would accept in the long run.
So, instead of bland unreality: To describe real gases, we have to take attraction and repulsion into account. Dutch physicist Johannes van der Waals wrote a simple but amazingly correct equation for real gas and liquid behavior in 1873 (p = RT/(v - b) - a/v2). He imagined an attractive force that dropped off with distance. But the only repulsion occurred when molecules collided like billiard balls.
By the 20th century we had a solid kinetic theory of gases. And we hoped to derive their physical properties from the molecules themselves. Of course, to do that, we'd need an accurate equation for the forces between molecules.
 Enter now, one John Edward Jones. Born in 1894, Jones studied math at Manchester, then joined the Royal Flying Corp. He flew during WW-I in those molecular biplanes that seemed to violently draw toward, then repel, one another. Following the war, in his Cambridge doctorate, he gave us the most durable equation for molecular forces. It was later called the Lennard-Jones potential. That was after he'd married Kathleen Lennard and added her name to his own.
Enter now, one John Edward Jones. Born in 1894, Jones studied math at Manchester, then joined the Royal Flying Corp. He flew during WW-I in those molecular biplanes that seemed to violently draw toward, then repel, one another. Following the war, in his Cambridge doctorate, he gave us the most durable equation for molecular forces. It was later called the Lennard-Jones potential. That was after he'd married Kathleen Lennard and added her name to his own.
The Lennard Jones equation is still in use today. By the time John Lennard Jones died at the age of only sixty, he'd done as much as anyone to put quantum mechanics to use in chemistry. And those contributions began with the need to understand the essential push-pull that underlies all things around us.
I'm John Lienhard at the University of Houston, where we're interested in the way inventive minds work.
For more on the use of the Lennard Jones Potential, see, e. g.. C. L. Tien and J. H. Lienhard, Statistical Thermodynamics. (Washington, DC: Hemisphere Publishing Corp. 1979, and other editions): Chapters 9 and 10.
See also the Wikipedia articles on John Lennard Jones and on his intermolecular potential function. J. Lennard Jones photo courtesy of Wikipedia Commons.
Some might be surprised at my assertion as to the accuracy of the van der Waals equation. On this matter, most writers are unaware of its enormous intrinsic validity when it is properly used. For more detail, see the papers in this location.