Bubbles in Soda
Today, bubbles in soda and bubbles in teakettles. The University of Houston's College of Engineering presents this series about the machines that make our civilization run, and the people whose ingenuity created them.
A scientifically savvy friend stopped me yesterday to ask if I'd ever done a program on champagne bubbles. He figured there must be more to them than meets the eye. And, indeed, there is.
We tend to think that bubbles in liquids should all be pretty much the same thing. In fact, bubbles that rise from carbonated liquids are completely analogous to those in boiling water, yet they're the result of radically different actions. Both are formed by something flowing through the liquid. But what does that mean? Liquids are supposed to do the flowing; and I'm asking you to picture something passing through a liquid while it just sits there.
One of those somethings is heat. For a vapor bubble to grow in a liquid, the surrounding liquid has to be above its boiling point. The liquid at the surface of the bubble stays right at its boiling point, so heat flows in from the hotter liquid around it. The heat flow sustains evaporation and the bubble grows. That process is very fast, by the way. Thirty or so bubbles can grow from one point at the bottom of your teakettle every second.
What flows in a carbonated drink is carbon dioxide gas. Carbonated drinks hold a lot of CO2 when they're cold and pressurized. They hold much less at lower pressures; so, when we pop the cork, the liquid becomes supersaturated with the gas.
That's a lot like a liquid being heated beyond its boiling point -- same idea. Heat flows from the superheated liquid to the bubble surface during boiling. Carbon dioxide flows from the supersaturated liquid toward the surface of a champagne bubble.
The big difference is that gas flows a lot more slowly than heat does. Of course you might not think so when you pop the cork, but that's because so many bubbles are released all at once. You'd face a far more vicious explosion if you overheated water as far as you typically over-saturate champagne with carbon dioxide.
Take something less extreme than uncorking a bottle. Take the glass of tap water that you put by your bed last night. Now, when you wake, its temperature has risen, just a little. You see bubbles on the side of the glass. They're filled with oxygen and other gases that're dissolved into any city water supply -- a little bit of nitrogen, and a varying amount of carbon dioxide. The bubbles in your glass grew very slowly while you slept. They might still be growing, but too slowly to be seen.
In both cases the bubble diameter increases roughly in propor-tion to the square root of time. But that growth stretches out over a much longer time for soda pop, champagne, or bedside water. Bubble growth is so fast in your teakettle that all you can make out is a feathery blur at the bottom.
By the way, if you re-cork champagne, more gas comes out of the solution and the pressure builds back up. But not as high. Much of the CO2 has been used up, and you'll never again get anything quite like that first pop from the bottle.
I'm John Lienhard, at the University of Houston, where we're interested in the way inventive minds work.
J. H. Lienhard, "Generalized Measures of the Stability of Bubbles Containing Permanent Gases," ASCE Hyd. Div. 12th Natl. Conf., Penn. State Univ., Aug. 1963.
A. Bhattacharya and J.H. Lienhard, "Similarity of Vapor and Gas Bubble Growth," Ir. J. Sci. and Tech., Vol. 1, No. 2, 1971, pp. 111-129.
Thanks to Dennis Clifford, UH Civil Engineering Dept., for additional counsel, and to pediatric oncologist Ken McClain for suggesting the topic.
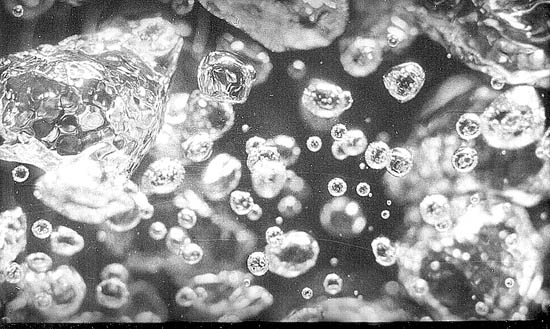
Water at a full rolling boil on a heated surface
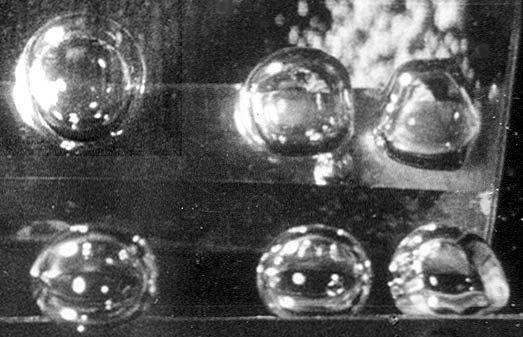
Two views of three bubbles growing, as water boils on a heated ribbon. Side view on bottom. Top view in the mirror above it. (Both photos by John Lienhard)