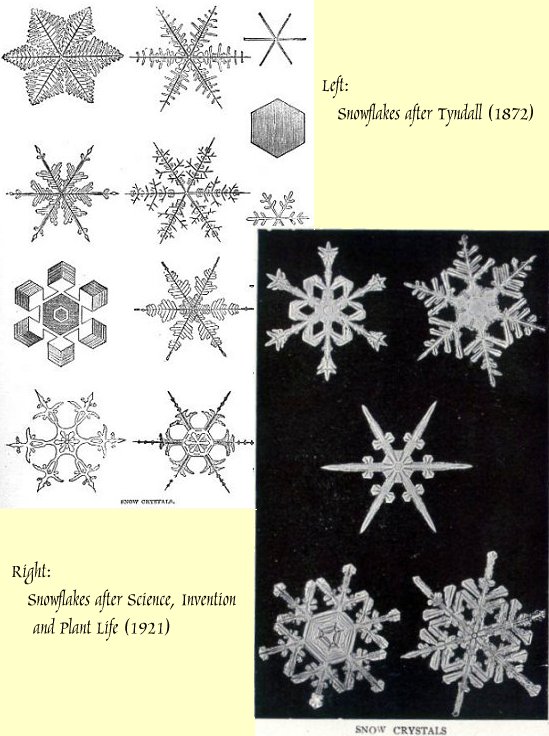
Snowflakes
Today, we shape a snowflake. The University of Houston's College of Engineering presents this series about the machines that make our civilization run, and the people whose ingenuity created them.
A delirious profusion of children's books on science appeared in the early nineteenth century. We'd invented fast rotary printing presses, and new kinds of cheap paper to feed them. Now education raced ahead of schools. Good schools were few and far between on the American frontier, but you could buy a cheap book.
And what books there were -- richly illustrated with prints and engravings of nature's beauty. It's not surprising that natural science books then, and ever since, have gravitated toward one of nature's beauties in particular -- the snowflake.
You and I know that snowflakes derive their beauty from the crystal structure of ice. But we were still debating the existence of molecules in the nineteenth century. We wouldn't analyze crystal structures with X-ray diffraction until the twentieth century.
In 1872, John Tyndall showed rare insight in a book on The Forms of Water. In it, he veered in his own description of snowflakes to explain how molecules must bond to one another. We didn't yet know the structure of ice, but he knew structure was there. And that structure determined the exquisite beauty of snow.
By 1919, one typical book for a younger audience says flatly that the crystal structure of snow is "a peculiar arrangement of the molecules of matter." That book included four pages of fine snowflake photos -- a dazzling variety of forms.
Now a new book: Librecht's and Rasmussen's, The Snowflake: Winter's Secret. Here, a physicist and a photographer take us to the heart of snowflake formation -- clear analysis combines with stunning photos. The frontispiece of the book is not one, but ten, pages of photos.
A snowflake is built on a crystal structure in the form of a hexagonal prism. It has six-sided symmetry, but only in one plane. Snowflakes grow, as molecules are attracted to points and cusps -- not to flat edges. Thus, a kind of fractal growing and splitting spreads outward to give snowflakes their remarkable forms.
The book shows how we can grow our own designer snowflakes. It also provides a map, showing how snowflake structures change with temperature and humidity. My Minnesota childhood reemerges as I trace that map. When I was a kid, I paid attention to the way weather affected my snowball-making. The snow at subzero temperatures was particularly delicate and lovely; but it would never cohere into a decent snowball.
Here in warm Houston, Texas, those varying forms of snow are all pretty academic. I breathe in the cool autumnal air of our January, and think about the Rubaiyat, where Omar Khayyam describes the fleeting snows of Persia:
The Worldly Hope men set their Hearts upon
Turns Ashes -- or it prospers; and anon
Like Snow upon the Desert's dusty Face
Lighting a little Hour or two -- is gone.
I'm John Lienhard, at the University of Houston, where we're interested in the way inventive minds work.
(Theme music)
K. Librecht and P. Rasmussen, The Snowflake: Winter's Secret Beauty. Stillwater, MN: Voyager Press, 2003.
J. Tyndall, The Forms of Water in Clouds & Rivers, Ice & Glaciers. New York: D. Appleton and Company, 1877. (Originally published in 1872.)
W. A. Bentley, The Treasures of the Snow. Science, Invention, and Plant Life (four editors) New York: The University Society, 1921. pp. 84-86. (My thanks to Margaret Culbertson, UH Art and Architecture Library, for this fine old book.)